Clinical Research
New Generation Sequencing
New Generation Sequencing
Applying the New Generation Sequencing (NGS) in the early stages of clinical trials makes it possible to significantly reduce risk in the next research phases. It is exactly because of the ability to select patients based on genetic biomarkers.
Genetic panels
In modern therapies, the targeted genetic panels have become common in the process of designing clinical study and patient selection. However, due to fast growing knowledge on the human genome, genetic panels do not allow to exploit it easily.
Human genome and microbiome
Pharmaceutical companies and their research teams seek for efficient ways to use data on the human genome and microbiome. Due to the nature and quantity of such data, it is expensive process, which implementation may take a long time.
Clinical Research
Clinical trials solutions
Databion Platform allows for correlating clinical results with genetic data of patients. The analysis results of the combined data make it possible to design clinical trial better and improve the patient selection criteria for individual phases, increasing safety and chances for successful outcome.
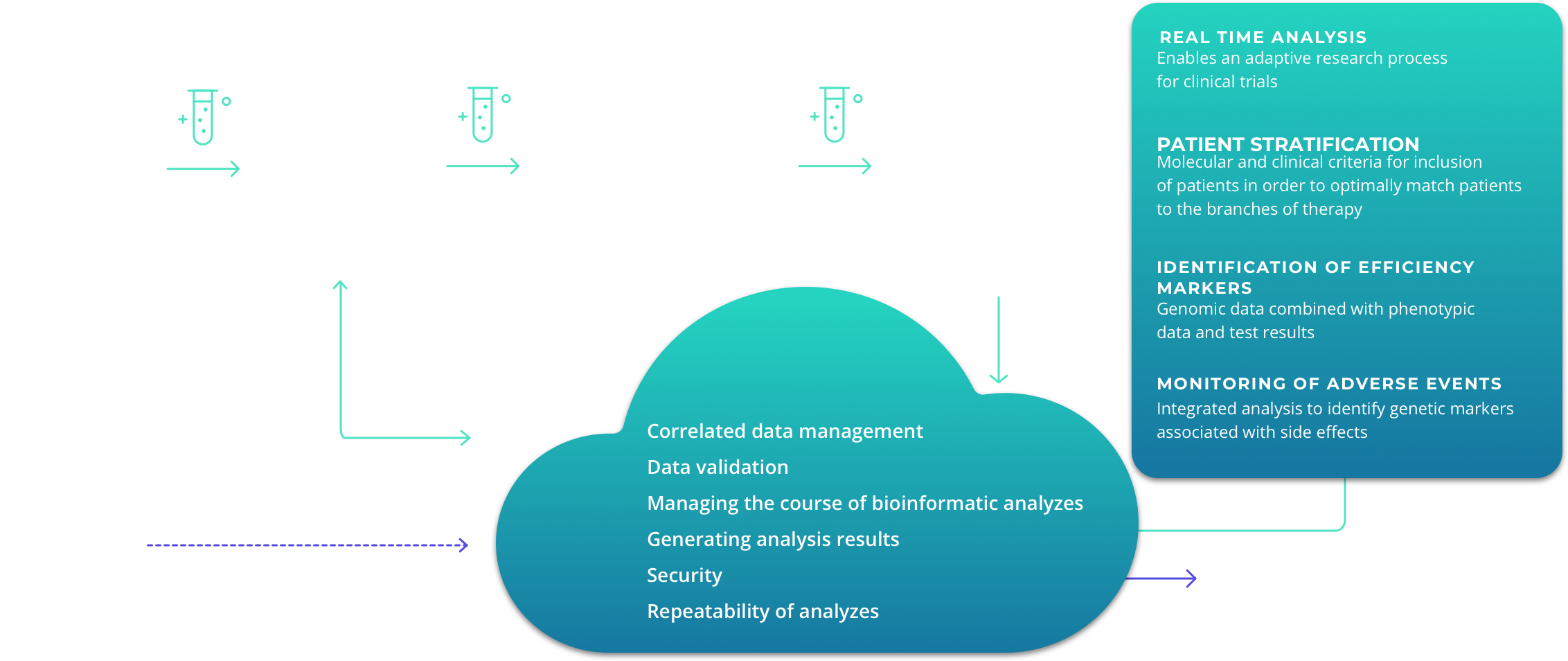
Clinical Research
Support in clinical research process
Phase 1
The first phase of clinical research aims to determine the early drug assimilability, specify pharmacokinetic and pharmacodynamic properties of the drug and define dosage. Genetic basis of reaction to a drug, determined at this stage of clinical research, may significantly increase chances of trials success.
Phase 2
The second phase of the clinical research is usually conducted on a group of a few hundred up to a thousand of patients. Selection rules of the group subjected to clinical trials are strictly defined and for example, the patients with comorbidities are eliminated. At this stage, the pharmacogenomics helps to establish relations between therapy effectiveness, patient's safety parameters, and the patient’s genetic profile. It also allows for better selection of patient cohort and determination of a drug dosage split by groups.
Phase 3
The third phase of the clinical research is the most expensive. Applying pharmacogenomics should ultimately allow for conducting broader, and more effective analysis of this phase. The key element here is to identify genetic markers in order to predict adverse reactions to a given drug. Both coding and regulatory regions of a gene should be taken into account.
Benefits
Databion in clinical trials
Databion allows to reduce time and costs of including NGS data in the clinical research process, improving results and minimising the risks of failure of the individual clinical trial phases.
predictable responses to
drug therapy,
particularly in chemotherapy
and severity of adverse
drug reactions (ADRs)
of drug discovery
and development
cost-effective clinical research